Influenza Vaccine Production : Influenza Vaccine Wikipedia
This method does not require an egg-grown vaccine virus and does not use chicken eggs in the production process. Cell-based refers to how the influenza flu vaccine is made.

Pandemic Influenza Vaccine Manufacturing Process And Timeline
The production of seasonal influenza vaccines is based on viral propagation in embryonated eggs or cell cultures.
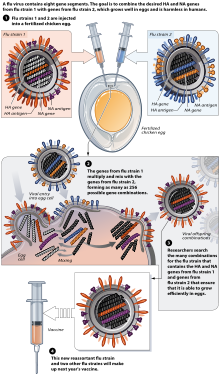
Influenza vaccine production. For the United States there are three different influenza vaccine production technologies approved by the US. Traditionally influenza vaccines are produced through the use of chicken eggs from qualified facilities in which viruses are grown and harvested. This method needs millions of eggs requiring orders to be placed about a year prior to production.
Vaccine bulk manufacture. Egg-based flu vaccine cell-based flu vaccine and recombinant flu vaccine. These projections may change as the season progresses.
We need to continually adapt production process to satisfy evolving regulatory demand which varies country by country. Once manufacturing activities begin egg-derived vaccines can take anywhere between 6 and 9 months to be produced before they are ready for distribution2. Four split and two whole inactivated virus WIV influenza vaccine bulks were produced and c.
For the 2021-2022 season manufacturers have projected they will provide as many as 188 to 200 million doses of influenza vaccine for the US. Most inactivated flu vaccines are produced by growing flu viruses in eggs. Influenza Vaccine Production and Design The most common method used to produce each years seasonal flu vaccine involves a laborious time-consuming process in which scientists must select vaccine strains months in advance of the upcoming flu season and then grow the selected flu virus strains in chicken eggs.
Although in potential the worldwide vaccine production capacity of 850 million doses per year 13 is nearly matching the seasonal demand for influenza vaccine this amount is not sufficient to cover demands for a pandemic outbreak. Seasonal strains of each type of influenza mutate and change very quickly so the flu shot is the only vaccine. Despite this setback production of seasonal flu vaccines continued.
While research towards developing universal influenza vaccines is ongoing the current strategy for vaccine supply in a pandemic relies on seasonal influenza vaccine production to be switched over to pandemic vaccines. Food and Drug Administration FDAexternal icon. These projections may change as the season progresses.
In the event of pandemic a pre-pandemic CVV can be used to. Flucelvax Quadrivalent is the only cell-based inactivated flu vaccine that has been licensed by the FDA for use in the United States. Experience in use of adjuvants.
Flu vaccine is produced by private manufacturers so supply depends on manufacturers. The aim of this study was to evaluate the impact of different inactivation and splitting procedures on influenza vaccine product composition stability and recovery to support transfer of process technology. Recombinant influenza vaccines are produced using recombinant virus technology.
Rapid development of reverse genetics technologies for generation of vaccine viruses. Increased development of mammalian cell lines for vaccine production. Flu vaccine is produced by private manufacturers so supply depends on manufacturers.
Vaccine manufacturers have projected that they will supply 188 to 200 million doses of influenza vaccine for the 2021-2022 season. In this review we have focused on conventional and novel methods for production of whole inactivated influenza vaccine. Outbreaks of influenza outside the typical season severe influenza among healthcare workers or clusters of vaccine failures that may herald novel influenza virus.
As well as chemical modification using formaldehyde or β-propiolactone and physical manipulation by ultraviolet radiation or gamma-irradiation novel approaches including visible ultrashort pulsed laser and low-energy electron irradiation are discussed. Sanofis recombinant influenza vaccine is called Flublok. The flu viruses used in the cell-based vaccines are grown in cultured cells of mammalian origin instead of in hens eggs.
Since 1990 there have been significant new developments in methods of influenza vaccine production. Vaccine manufacturers have projected that they will supply the United States with as many as 188 million to 200 million doses of influenza vaccine for the 2021-2022 season. Influenza Vaccine Manufacturing Infrastructure Multiple new influenza vaccine products and manufacturing technologies are becoming available This creates an opportunity to bypass outdated technology as has been done with cellular telephone technology Investments should result in sustainable capabilities.
Vaccines will be an important element in mitigating the impact of an influenza pandemic. H Virological Provide candidate vaccine viruses CVV for seasonal and pre-pandemic influenza vaccine production. The production of a vaccine can take between 6 and 36 months Vaccines manufacturing is a biological process where a very high level of expertise is required.
Since vaccine production takes about 6 months each year the influenza vaccine is produced under great time pressure requiring timely submission of viruses to the WHO GISRS. The vaccine virus is injected into thousands of eggs and the eggs are then incubated for two to three days during which time the virus multiplies. Projections may change as the season progresses.
Flu vaccine is produced by private manufacturers so supply depends on manufacturers. For most influenza vaccine production this is performed in nine to twelve-days old fertilized hens eggs.

Weathering The Influenza Vaccine Crisis Nejm
Cilian Ag The Ciliate Vantage For Biopharmaceuticals
Bird Flu Vaccine Production Processes Infographic

Inactivated Flu Vaccine Vaccine Knowledge

Better Influenza Vaccines An Industry Perspective Journal Of Biomedical Science Full Text

Flu Vaccine Production Process For The Southern Hemisphere Youtube

Current And Next Generation Influenza Vaccines Formulation And Production Strategies Sciencedirect

Making The Flu Vaccine A Race Against The Clock Sanofi

A Tale Of Two Mutations Beginning To Understand The Problems With Egg Based Influenza Vaccines Sciencedirect

5 The Yearly Influenza Vaccine Production Timeline Begins In January Download Scientific Diagram

A Cell Based Backup To Speed Up Pandemic Influenza Vaccine Production Sciencedirect

Egg Based Influenza Vaccines Esco Vaccixcell
Gsk Flu Vaccine Production Process Pdf

Timeline Of Current Influenza Vaccine Production Methods Schematic Download Scientific Diagram

Timeline Of Current Influenza Vaccine Production Methods Schematic Download Scientific Diagram

Hassle Free Influenza Vaccine Close To Reality

Egg Based Pilot Influenza Vaccine Production Process Download Scientific Diagram